Longevity for pets: from zero to one
Targeting deregulated nutrient-sensing and mitochondrial dysfunction to bring the first product to market that increases healthy lifespan in companion animals

In the previous post I explored some physical and functional changes that companion animals go through as they age. Mostly to understand how good of a model they are for studying aging in people.
Going deeper, it seems that some molecular and cellular ageing mechanisms are consistent across distantly related species. I want to go over some of them; specifically the ones related with nutrient sensing (the ability of cells to adjust their metabolism to the amount of nutrients available) and mitochondria (the powerhouses of the cell).
I think it’s important to explore this topic, especially at this point in time, because most companies and organizations trying to productise aging therapeutics for companion animals are targeting these mechanisms. And it could be a strategic way to move the space from zero to one: first for them; and then for us.
Here I focus on compounds already studied specifically in companion animals; which are not that many. There is more research on simpler models like yeast, worms, flies, and mice that could apply to dogs, cats, and horses. This post aims to show how some compounds affecting simple cellular mechanisms could modulate aging in companion animals and extend healthy lifespan.
IGF-1
Soon Loyal is starting a pilot study for LOY-002, their daily tablet intended to extend lifespan and quality of life for older dogs. Cleo Abram already made a great video about Loyal and you should watch it now. It’s a masterclass on communication.
Here I want to go deeper and talk about how their drugs probably work. For educational purposes only (my education, not yours), let me focus on LOY-001: the drug they started with to figure out how to quantify healthspan in dogs.
Some time ago Celine Halioua (Loyal’s CEO) wrote about her interest in a study in mice treated with Insulin-like Growth Factor 1 receptor (IGF-1R) inhibiting monoclonal antibodies. Which matches the description of their product LOY-001: long-acting product given to dogs every three to six months. Why would that increase healthy lifespan in dogs?
LOY-001 targets nutrient sensing, a well-studied molecular and cellular mechanism that impacts healthy lifespan. Specifically, it targets IGF-1, a hormone responsible for stimulating cell growth, division, and differentiation. And more specifically it targets the receptor (IGF-1R) responsible for binding it and mediating its effects.
IGF-1 belongs to a group of nutrient-sensing signalling pathways that allow cells to sense and respond to nutrient availability, like glucose and amino acids. These pathways have evolved to synchronise cell growth and metabolism with nutrient availability.
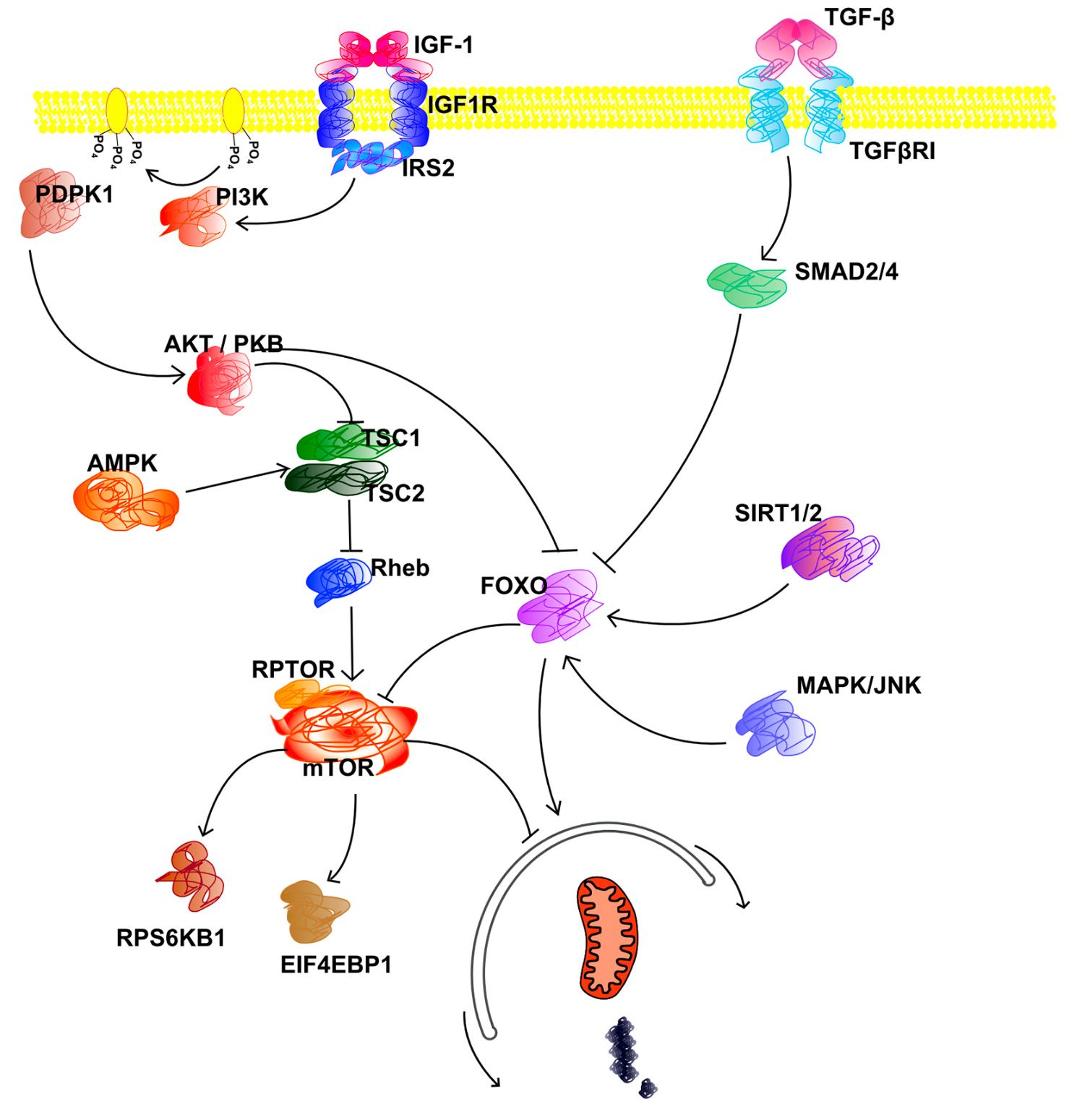
One fascinating aspect of IGF-1 is how a single mutation in its gene has a massive impact on size variation in dogs. This mutation accounts for up to 50% of size variation across all breeds.[1] Increased IGF-1 signalling in large breeds not only influences growth and development but also likely boosts protein synthesis by mTOR, which is also a critical mechanism in aging biology. We know that large-breed dogs have a shorter lifespan and life expectancy than small-breed ones.[2] So it's possible that the IGF-1 allele responsible for larger size could also be responsible for lower lifespan. It could be an example of antagonistic pleiotropy (when a gene has both beneficial and detrimental effects).
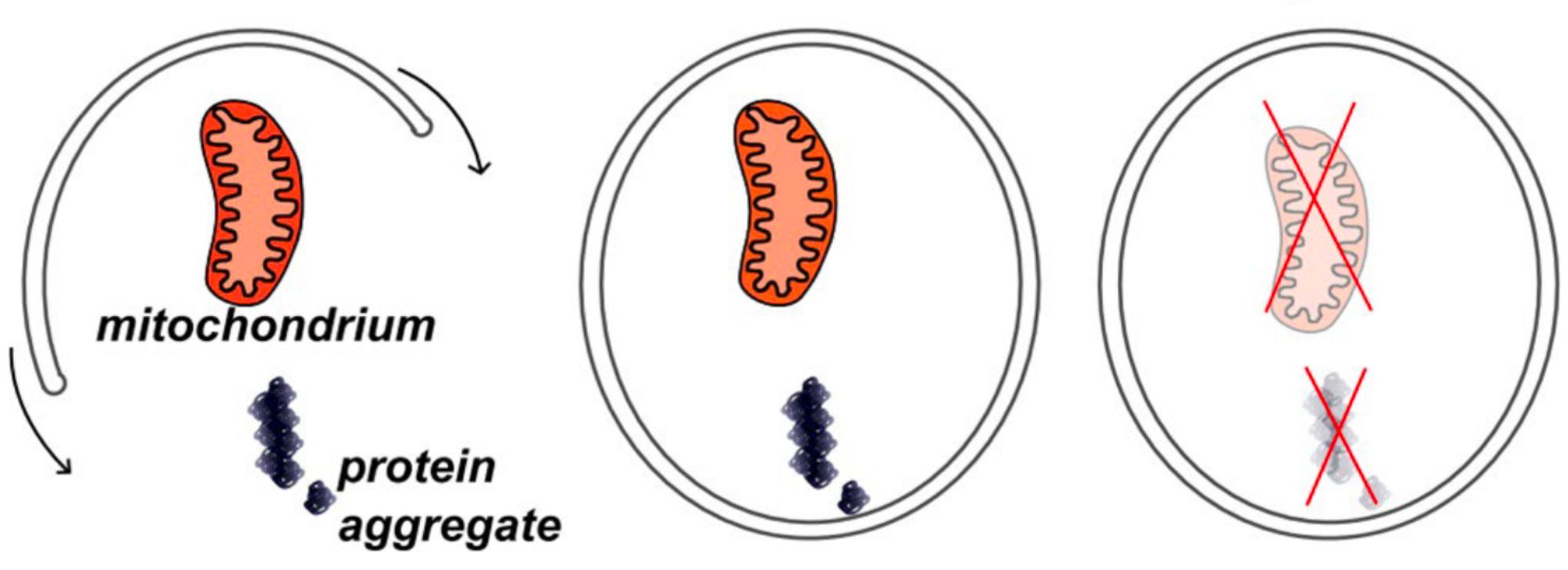
Inhibiting IGF-1 signalling could mimic the effects of caloric restriction, causing a metabolic shift that prioritises maintenance and repair over cellular reproduction. This change activates autophagy, a cellular process that eliminates and recycles damaged proteins and organelles, effectively revitalising cells. Autophagy is essential for maintaining cellular health and preventing the buildup of damage associated with aging.
It is a good hypothesis because:
- it has been shown that caloric restriction and fasting increase lifespan in model organisms.[3]
- in elderly women (95 years), as well as in a mixed population of older adults (mean age 76 years), low IGF-1 levels correlate with a low probability of cognitive impairment and death.[4]
For some reason Loyal changed their strategy and the first drug they are pushing to market will be a daily tablet. And I don’t know if it’s inhibiting IGF-1 signalling. We'll see.
mTOR
There are other ways to prioritises maintenance and repair over reproduction besides inhibiting IGF-1 signalling.
Most nutrient sensing mechanisms converge or interact with a common target, the mammalian target of rapamycin (mTOR) kinase, which integrates information from these pathways to regulate protein synthesis and cellular metabolism. This convergence is crucial for coordinating various cellular processes related to growth and metabolism.
Rapamycin (also known as sirolimus) is a specific inhibitor of mTOR. It is an FDA-approved drug that has been used for many years to prevent organ transplant rejection. It is also the most promising known compound that could extend healthy lifespan. Depending on the dose and the delivery regimen, rapamycin extends lifespan in yeast, worms, flies, and multiple mice models.[5]
Loyal are not the only ones who care about extending healthy lifespan in companion animals. The Dog Aging Project is trying to understand how genes, lifestyle, and environment influence aging in dogs. They already did a randomized controlled trial of 24 middle-aged companion dogs administering a low-dose rapamycin weekly treatment. They showed that it was safe over a period of 10 weeks and observed some of the same beneficial effects that have been previously shown for mTOR inhibition in mice. Rapamycin can be safely administered to companion dogs with at least the potential for significant health benefits.[6] Based on those results they are starting a larger double-blinded, placebo-controlled clinical trial in dogs (TRIAD) to determine if this drug extends healthy lifespan: extend lifespan but also to ensure a higher quality of life during those additional years.
These nutrient sensing pathways get dysregulated which affects processes beyond metabolism: resistance to stress, activation of repair mechanisms, autophagy stimulation, or inflammation control.[7] And because mTOR is a central piece of this network, Trivium Vet is targeting several age-related diseases in dogs and cats using rapamycin. They are starting a study in dogs with dilated cardiomyopathy (DCM), a condition where the heart becomes enlarged and weakened, impairing its ability to pump blood efficiently. Dogs with DCM will receive rapamycin along with their other cardiac medications. Additionally, they are conducting a proof-of-concept trial using a delayed-release formulation in cats with hypertrophic cardiomyopathy (HCM), a condition where the heart muscle becomes too thick.
Trivium Vet is also starting a study on cats with chronic kidney disease (CKD), a progressive condition where the kidneys lose their ability to function effectively over time. Aging of the kidney may contribute to the onset and progression of CKD. Finally some some good news for Mooncake 🐈 and Freya 🐈⬛.
Trivium Vet is targeting diseases related to aging but not aging directly. In my opinion, the testing of anti-aging drugs should focus on their effectiveness in addressing all-cause aging, rather than targeting specific diseases. Only that way we can push the longevity market (the real one) from zero to one.
AMPK
While mTOR promotes cell growth and metabolism when nutrients are available, AMP-activated protein kinase (AMPK) serves as its counterbalance. When energy levels are low, AMPK is activated, and it can inhibit mTOR. Both communicate to make sure the cell uses its resources wisely, adjusting to the cell's energy situation. Some compounds have been shown to activate AMPK in dogs and horses like AICAR or metformin.
AICAR is a compound that stimulates AMPK and is primarily used as a research tool in laboratories for investigating the effects of AMPK activation. It has shown potential in improving athletic performance, protecting the heart, and regulating glucose and lipid metabolism. AICAR is not currently approved for any specific medical use in people or animals. Its potential performance-enhancing effects have led to concerns about its use as a doping agent in sports. The World Anti-Doping Agency (WADA) has included it on its list of prohibited substances to prevent its misuse in competitive sports.
Metformin is a widely used medication for managing diabetes in humans. It works by reducing glucose production in the liver and increasing the sensitivity of muscle cells to insulin, helping the body use glucose more effectively. In recent years, metformin has gained attention for its potential role in promoting health and longevity in animals, including dogs and horses, by activating AMPK and improving insulin sensitivity.
In dogs, both metformin and AICAR are known to activate AMPK, which helps protect the heart against damage caused by reduced blood flow and its subsequent restoration. Metformin has been shown to decrease the death of heart muscle cells and slow down the progression of heart failure in dogs. AICAR has demonstrated similar effects to metformin, suggesting that activating AMPK plays a major role in protecting heart cells and preventing heart failure.[8] A study found that a low dose of AICAR increased glucose uptake in the muscles of dogs. These effects were influenced by whether or not the body had previously been blocked from breaking down fats for energy. This suggests that AICAR may also affect how dogs process glucose and choose fuel sources, which could have potential implications for aging and longevity.[9]
In horses, metformin has shown promise in reducing blood glucose and insulin levels in both healthy horses and those with experimentally induced insulin resistance.
10 It also helps decrease the risk of laminitis11, a painful and debilitating hoof condition in horses linked to aging.12 AICAR, on the other hand, has been found to promote glucose uptake in horse muscle through AMPK activation. It has also been suggested that AICAR enhances insulin secretion from the pancreas.13 Collectively, these findings imply that activating AMPK with metformin and AICAR could be a valuable therapeutic strategy for managing insulin resistance, reducing the risk of laminitis, and promoting overall health and longevity in horses.
As with rapamycin, it could be that, depending on dose and delivery regimen, compounds that activate AMPK could extend healthy lifespan and delay age related diseases.
Mitochondria
AMPK plays a critical role in maintaining mitochondrial health. Mitochondria are the parts of the cell responsible for creating energy through a process called cellular respiration. During cellular respiration, oxygen interacts with nutrients, like glucose and fats, to produce energy in the form of ATP (adenosine triphosphate).
When AMPK detects low levels of ATP, it responds by regulating the removal of damaged or dysfunctional mitochondria (mitophagy) and the formation of new mitochondria (mitochondrial biogenesis). This means AMPK could impact the aging process by either promoting or inhibiting mitochondrial function.[10]
As a byproduct, mitochondria also produce reactive oxygen species (ROS). While low levels of ROS are normal and play important roles in cellular signalling, an excess of ROS can be harmful, because they can damage important cellular components like proteins, lipids, and DNA. ROS can also damage the mitochondrial genome. Accumulation of mutations in the mitochondrial genome may lead to aberrant mitochondria that consequently produce even more elevated rates of ROS. Removal of aberrant mitochondria is a key process for maintaining oxidative balance in cells.

In older dogs, regardless of size, both energy levels and important antioxidants like glutathione appear to be lower. This implies that increased ROS and reduced energy production play a role in the aging process for all dogs. However, besides IGF-1, there are many differences in the metabolism of small and large breeds, which could be the key to understanding the significant variation in their lifespans.
In long-lived small dog breeds, their mitochondria produce energy more efficiently and generate less cellular waste. Which may contribute to a lower risk of cellular damage, a more robust response to stress, and ultimately, a longer lifespan. In contrast, large dog breeds show increased glucose breakdown rates and more DNA damage, which could be the reason for their shorter lifespans compared to small breeds.[11]
Carnitine, a molecule derived from amino acids, transports fatty acids into mitochondria for energy production. As they age, large dogs struggle to process glucose efficiently and rely on an amino acid called glutamine for energy, while small dogs show an age-related increase in carnitine, essential for using fats as an energy source. In older dogs, large breeds tend to have lower carnitine levels than small breeds.[12]
Astaxanthin, a natural pigment with strong antioxidant properties, improves mitochondrial function in dogs of all ages by reducing cell damage. This suggests that astaxanthin supplementation could help maintain dog health and slow aging.[13]
These and other metabolic differences, not necessarily related to mitochondria, could help us understand why size affects how dogs age. For example, small dogs have higher levels of substances related to tryptophan, an essential amino acid involved in many metabolic processes like serotonin and melatonin production.[14]
Maybe, and only if proved with the rigour of a randomized controlled double blind study, some supplements could be brought to market as a product extend healthy lifespan in companion animals.
Dysfunction of the mitochondria has been observed in many age-related diseases in older horses, dogs and cats.
In older horses, skeletal muscles experience a decline in mitochondrial content and function.[15] This decline can be attributed to reduced biogenesis and compromised mitophagy. Genes responsible for creating new mitochondria appear to be more active in younger horses. Older horses also show reduced levels of certain proteins related to autophagy, indicating that the processes of breaking down and recycling damaged cellular components might be less efficient.[16]
Laminitis has been linked to mitochondrial dysfunction. Its primary cause is believed to be energy deficiency because in the acute phase of laminitis, muscle mitochondrial respiration decreases, regardless of the cause (metabolic disorder or systemic inflammation). This decline in mitochondrial function could lead to a depletion of the cell's ATP content, which in turn might contribute to the progression of laminitis. It could be that the pathology is triggered after a mitochondrial dysfunction threshold is reached.[17]
The heart ♥️ is the most active metabolic muscle. Each cardiac muscle cell contains approximately 5,000 mitochondria; the most of any tissue. Normal cardiac function depends on high energy supply in the form of ATP.[18] That’s probably why many abnormalities in mitochondria have been observed in cardiac diseases in dogs and cats.
In dogs, chronic heart failure (HF) has been associated with abnormalities in heart mitochondria, including changes in size and structure, contributing to overall heart dysfunction. It seems that dogs with chronic heart failure have impaired mitochondrial respiratory activity, resulting in reduced energy production.[19] Elamipretide (also known as Bendavia), a mitochondria-targeting peptide, has demonstrated promising results in addressing these issues.[20] After long-term therapy with Elamipretide, mitochondrial dynamics in heart failure cases have returned to normal, suggesting its potential as a treatment option and as an aging therapeutic.[21]
Stealth BioTherapeutics is currently doing several studies in people with Elamipretide for several age-related conditions where mitochondrial dysfunction has been observed. Maybe Elamipretide can be brought to market as an aging therapeutic for dogs?
Mitochondrial dysfunction has been shown to play a crucial role in feline hypertrophic cardiomyopathy (HCM), a heart condition in which the muscle becomes abnormally thick. The condition involves a decrease in the heart's ability to produce energy through a process called cardiac mitochondrial oxidative phosphorylation (OXPHOS).[22] A study on domestic cats with HCM discovered that their heart cells had impaired OXPHOS capacity, leading to increased oxidative stress.[23] Given how close is the connection between mitochondria and the rest of the nutrient sensing pathways, that's probably why Trivium Vet is targeting HCM in cats with rapamycin.
As with rapamycin and AMPK activators, it could be that, depending on dose and delivery regimen, compounds that target the mitochondria (either directly or indirectly) could extend healthy lifespan and delay age related diseases.
Takeaways
Loyal and Trivium Vet are among the few companies trying to use a known compound to target a single aging mechanism or process. And it turns out that nutrient sensing is among the best studied mechanisms in aging biology. And it is highly conserved across distantly related species. So what could work for companion animals, could work for us too. It wouldn’t extend healthy lifespan indefinitely. But it could be the first step in this long journey.
These companies are at what I call Level 1. The biggest challenge for Level 1 (and Level 2) is that there are no proxy biomarkers for extending lifespan. At least none that the FDA and the scientific community agree on. Recently a Consortium has been created to establish reliable biomarkers of aging for longevity interventions but the timeline is not certain. The space needs new models for running clinical trials. Running them in dogs like Loyal and The Dog Aging project are doing may be just the right strategy. At least for now.
I think companies at Level 1 are the most likely to be the first ones to bring an aging drug to market. They will be the ones to prove how promising is the longevity biotech market. They will become experts in collaborating with regulators, will create the first distribution channels and will become models in the space creating new playbooks on how to start and manage longevity biotech companies.
This is a complex topic and any attempt to compress it into a digestible post may look like an oversimplification. If you want to go deeper, below you can find some papers that helped me write this post.
A Single IGF1 Allele Is a Major Determinant of Small Size in Dogs ↩︎
Lifespan of companion dogs seen in three independent primary care veterinary clinics in the United States ↩︎
Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms ↩︎
Insulin-like Growth Factor-1 and IGF Binding Proteins Predict All-Cause Mortality and Morbidity in Older Adults ↩︎
The Target of Rapamycin Signalling Pathway in Ageing and Lifespan Regulation ↩︎
A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs ↩︎
Impact of in vivo fatty acid oxidation blockade on glucose turnover and muscle glucose metabolism during low-dose AICAR infusion ↩︎
AMPK: guardian of metabolism and mitochondrial homeostasis ↩︎
Cellular energetics and mitochondrial uncoupling in canine aging ↩︎
Metabolomics of aging in primary fibroblasts from small and large breed dog ↩︎
Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dog ↩︎
Tryptophan metabolism is differently regulated between large and small dog ↩︎
Effects of aging on mitochondrial function in skeletal muscle of American Quarter Horse ↩︎
Skeletal muscle from aged American Quarter Horses shows impairments in mitochondrial biogenesis and expression of autophagy markers ↩︎
Muscle Mitochondrial Dysfunction in Horses Affected by Acute Laminitis ↩︎
Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cell ↩︎
Abnormal Mitochondrial Function in Myocardium of Dogs with Chronic Heart Failure ↩︎
Abnormalities of Mitochondrial Dynamics in the Failing Heart: Normalisation Following Long-Term Therapy with Elamipretide ↩︎
Why All the Fuss about Oxidative Phosphorylation (OXPHOS)? ↩︎
Impaired cardiac mitochondrial oxidative phosphorylation and enhanced mitochondrial oxidative stress in feline hypertrophic cardiomyopathy ↩︎